Malignant melanoma today: where are we now and where will we go? Part. 4
| 30 June 2022
0 Replies
DESCRIPTION
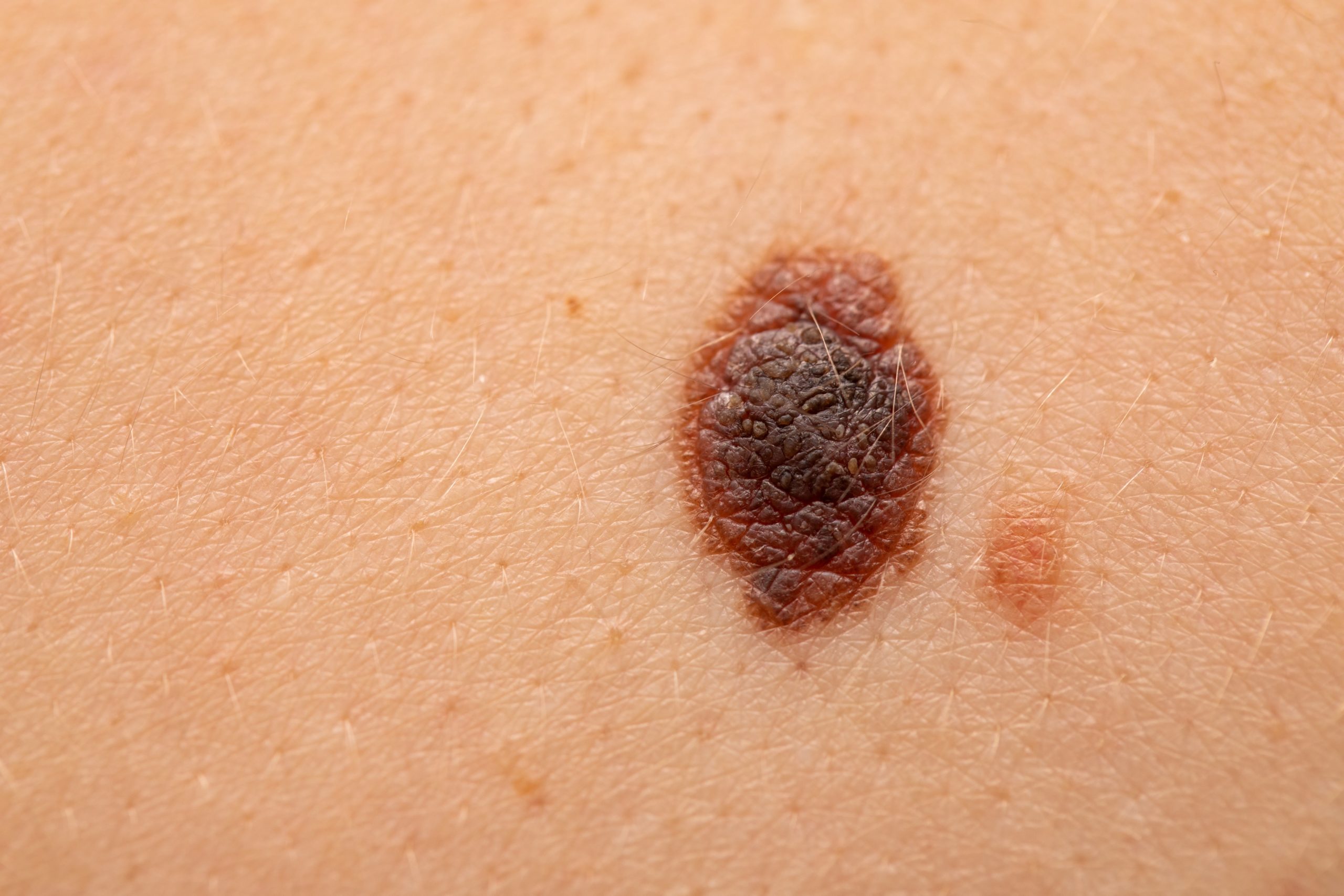
Malignant melanoma (MM) is known in the medical literature as the "Great Mime" of pathological anatomy, as it can simulate, in different ways, other neoplasms, both of an epithelial nature and of a mesenchymal nature.
From the point of view of location, MM may originate de novo on healthy skin or represent the malignant transformation of a pre-existing melanocytic nevus.
In the vast majority of cases, MM represents a sporadic event, while in less than 10% it is linked to alterations of tumor suppressor genes (encoded by the chromosomal region 9p21) and shows a hereditary character, for this reason it is defined as 'familial’.
Histopathologically, the MM of the skin and/or mucous membranes consists of neoplastic melanocytic elements, fused or epithelioid, with the presence of atypia and, often, mitotic figures. An accurate histological diagnosis is the basis for a clinical management of the patient affected by MM, since all the histopathological parameters reported in the report have important implications not only from a diagnostic but also from a prognostic point of view.
Histological Report of Malignant Melanoma
In the first instance, it is very important to determine whether the lesion we are analyzing constitutes an MM and is properly differentiated from atypical pigmented lesions that can closely simulate MM.
Morphologically the cutaneous MM can be classified into four histological subtypes such as: superficial spreading type, lentigo maligna, acral lentiginous and nodular type.
If at first, we tended to think that this was a mere histological description, in fact, more recent studies have correlated (sometimes very exhaustively) histological subtypes with particular molecular signatures of MM. For example, you can easily appreciate from the last WHO blue book "Classification of Skin Tumour"2018, IARC, that melanomas on skin chronically exposed to the sun have different chromosomal aberration patterns than melanomas that arise on skin with intermittent exposure to ultraviolet (UV) rays or in areas of acral skin or mucous membranes.
Therefore, a correct histological recognition of the MM subtype is of great importance and must always be reported in the pathological report of an MM.
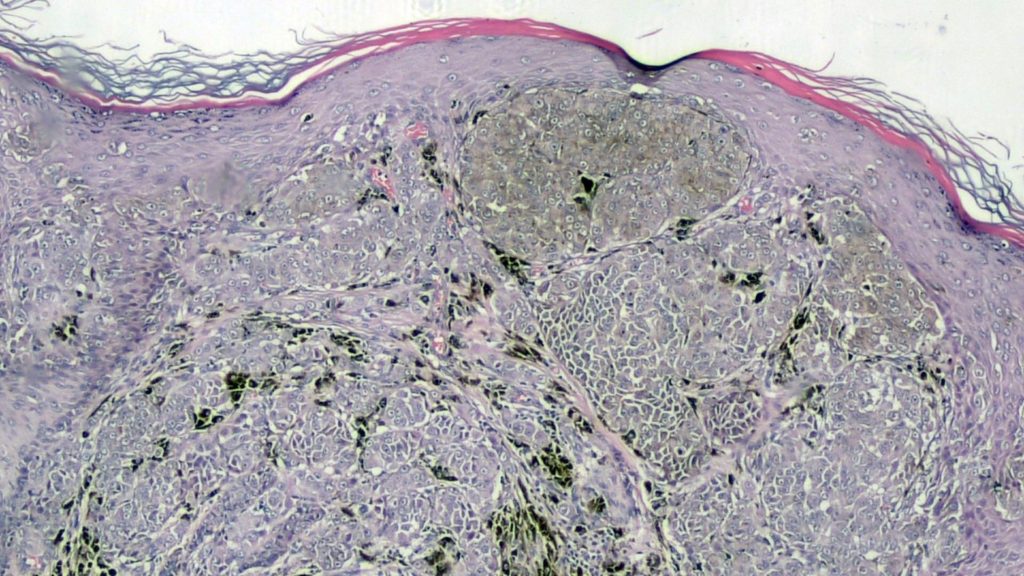
Figure 1 shows an example of MM with features consistent with ‘Spitzoid’ MM. Note that the lesion is composed by nests of melanocytes with some mitotic figures; top left is possible to appreciate pagetoid spreading of single melanocytes (another useful clue to diagnosis of MM).
Thickness according to Breslow it is the strongest and most reliable prognostic factor in MM and is defined as the measurement of the vertical thickness of the neoplasm.
The American Joint Committee on Cancer (AJCC) 8'Edition criteria for staging accurately predict sentinel lymph node positivity in clinically melanoma negative lymph node patients.
In fact, when grouped by AJCC cutting points, there was an increased incidence of positive sentinel lymph nodes with increasing tumour thickness: 4% in melanomas below 1.00 mm, 12% in melanomas 1.01-2.00 mm, 28% in melanomas 2.01-4.00 mm and 44% in melanomas over 4,00 mm.
But it is important to consider that there are, however, cases in which the thickness of Breslow does not impact perfectly on the prognosis: it is the case of thin melanomas that are able not only to metastasize, but also to bring the subject to death with some ease.
By convention, the thickness of Breslow is measured from the granulous layer to the last cell of MM, except in the case of ulcerated lesions, where the base of the ulceration is taken as a reference to the last point where the neoplasm is evident.
A parameter related to the thickness of Breslow is the Clark level, which, unlike the first, is a topographical criterion, which is based simply on the definition of which area of skin is affected by MM: superficial (or papillary) dermis, reticular, subcutaneous.
In recent years, the low reproducibility and repeatability of this parameter, due to its rather 'subjective' nature of measurement, has in fact caused its disuse, until it is no longer considered mandatory to be included in a histopathological report of melanoma.
However, we still consider it correct to include this finding in the MM report as an additional indicator parameter, never to be substituted for Breslow.
Another important element that can never be missing in the description of a melanoma is the number of mitoses / mmq. In fact, various studies have shown how the number of mitoses can be a rather reliable indicator of the biological behavior of a given MM (staging and prognosis).
Although there is no universally accepted method for counting mitotic figures in melanoma, the AJCC 8'Edition recommends making this measurement in the "hot spot" areas of the lesion, so as to ensure a faithful count of the maximum number of mitoses present.
It’s very important to consider one parameter on what there’s still no precise agreement in the literature, which is the regression of melanoma.
In fact, among the various theories, the most accredited considers the regression as a result of aggression of melanoma cells by the immune system with the substitution of the same with fibrosis, melanophages, lymphocytic infiltrate and angiectasis. Regression phenomena can be from focal to extended until the complete disappearance of the primary lesion (so-called 'burn-out melanoma').
The difficulty of reproducibility of this parameter consists in the variability of what is considered as regression: some studies have considered regression as the total absence of melanoma cells, whereas other studies have also considered more or less partial regression areas in the measurement of regression proper. Regression is now considered to be an independent prognostic factor for worsening prognosis, and according to the College of American Pathologists (CAP) guidelines, regression is measured by cutoff at 75%.
An integral part of a histological report of MM is the evaluation of ulceration, considered an independent prognostic factor for survival associated with melanoma.
Ulceration is defined as a continuous 'true' solution at full thickness, of the epidermis, with the presence of fibrin deposition or neutrophil granulocytes and/or reactive hyperplasia of the surrounding epidermis in the absence of trauma or surgical manipulation prior to or in progress.
In recent years more and more evidence supports the need to express the radial extent of ulceration (in mm), as it would seem that more extensive ulcerations correspond to reduced survival values. It should also be remembered that it is necessary to be sure of the presence or absence of this parameter, as over-staging of the MM in question can affect the entire diagnostic-therapeutic-care path of the patient.
Lymph vascular invasion (LVI) is a very useful parameter in predicting possible disease diffusion in a metastatic setting. For example, now included in the AJCC 8'Edition, this parameter has been shown to significantly increase the risk of recurrence, lymph node involvement, distant metastases and death, just like the ulceration parameter. From a histopathological point of view, consideration should be given to the possibility that tissue artifacts can simulate and/or obscure the morphology of lymphatic and/or blood vessels, and, therefore, studies have been carried out which have shown that the use of immunomarkers such as D2-40 (Podoplanin) can help to evaluate true lymph-vascular permeations that could otherwise give false-negatives.
Perineural invasion (PNI) is defined as the infiltration of nerve fibers by melanoma cells, resulting in an extension of the tumor along the surrounding nerves.
This parameter is of some importance and should be included in the histological report of the MM, as it (from the literature examined) is a pejorative prognostic factor. In addition, it is good to remember that there are some types of melanoma that are more easily neurotropic, such as desmoplastic melanoma or fused cell melanoma, and, therefore, this aspect is to be considered when diagnosing one of these types of lesions. Indeed, the high local recurrence rate of desmoplastic or fused-cell melanoma requires more aggressive surgery to reduce this risk.
Microsatellites are defined in the current CAP recommendations as microscopic and discontinuous cutaneous and/or subcutaneous metastases having a diameter larger or equal to 0.05 mm in largest dimension, adjacent to a primary melanoma. The presence of microsatellites increases from 4.6% in tumors less than 1.5 mm to 65% in those greater than 4 mm.
Furthermore, microsatellites are also associated with an increased frequency of regional lymph node metastasis in tumors greater than 1.5.
Lymphocytes infiltrating the tumor (TILs) are a type of lymphocytes capable of attacking neoplastic cells and, depending on the mode and distribution, are divided in ascending order into: absent, non-brisk or brisk. Several studies tend to point out that an increase in TLL’s would be more correlated to an improvement in survival, but also in this area (similar to what we have seen for regression) inter-observer variability does not help to obtain a clearer concordance of studies.
Subtypes of Malignant Melanoma
Melanoma can be considered in the radial growth phase (when melanomatous cells grow in the horizontal plane without giving rise to a vertical growth phase) and in the vertical growth phase, in turn divisible into tumorigenic (nests/cases deeper than the surface) or non-tumorigenic (melanocytes that do not form a net lesion). It is also very important to consider that melanoma in situ (by definition limited to the basal membrane, therefore pTis according to AJCC 8'Edition) is different from a melanoma in radial growth phase that, if not called 'in situ', suggests the possibility of the presence of some melanocyte minutes present below the basal membrane (c.d. microinvasive).
Superficial Spreading Type Melanoma (SSM)
Superficial spreading melanoma is the most frequent histotype in the Caucasian population (70-80% of all melanomas). Usually, it is localized to the back in men and to the lower limbs in women.
It’s the most common type of melanoma in patients with dysplastic nevus syndrome, with familial melanoma and multiple melanomas. Histologically it is characterized, in the intraepidermal component of the horizontal growth phase, by the presence of pagetoid cells (Figure 2) and by a proliferation, in the dermal area, of neoplastic cells frequently associated epitheliod type often to other cytotypes. In many cases it can observe a horizontal intraepithelial proliferation of the dysplastic nevus type. Atypical cells single or in small nests can be observed throughout thickness of the epidermis arranged in an irregular way up to the most superficial layers.
At the beginning, the phase of horizontal growth is characterized by invasion of the papillary dermis by single cells or small cell nests. Parallel to the initial invasion, the appearance of a marked inflammatory lymphocytic reaction can be noted.
There may be scattered macrophages mixed with inflammatory infiltrate, fibroplasia and vascular neogenesis.
The atypical cells present in the most superficial portion of the papillary dermis, in the horizontal growth phase, have the same cytological characteristics of the cells present in the epidermis and also the nests of cells are similar in size to those present in the epidermis. In the horizontal growth phase, no cell nest present in the dermal area it presents prevailing characters compared to the other nests adjacent.
The cells of the vertical component on the other hand, they have different cytological characters and are predominantly epithelioid-type cells aggregated in nests with cohesive characters.
Often a prevailing and expansive type of growth with a tendency for cell proliferation to develop in a centrifugal direction.
The nests of atypical cells which form the vertical growth are in size greater than intraepithelial ones.
The nuclei are bulky, hyperchromatic and polymorphic with prominent nucleoli and irregular nuclear membranes.
An always evident feature is the lack of cellular maturation with cells with identical cytological features both in the most superficial portion and in the deepest portion of the vertical phase. Mitoses can be in number variable, sometimes even numerous, but above all mitosis is also present in the plus portion deep melanoma. Isolated cells or groups of necrosing cells are often seen in the dermal component.
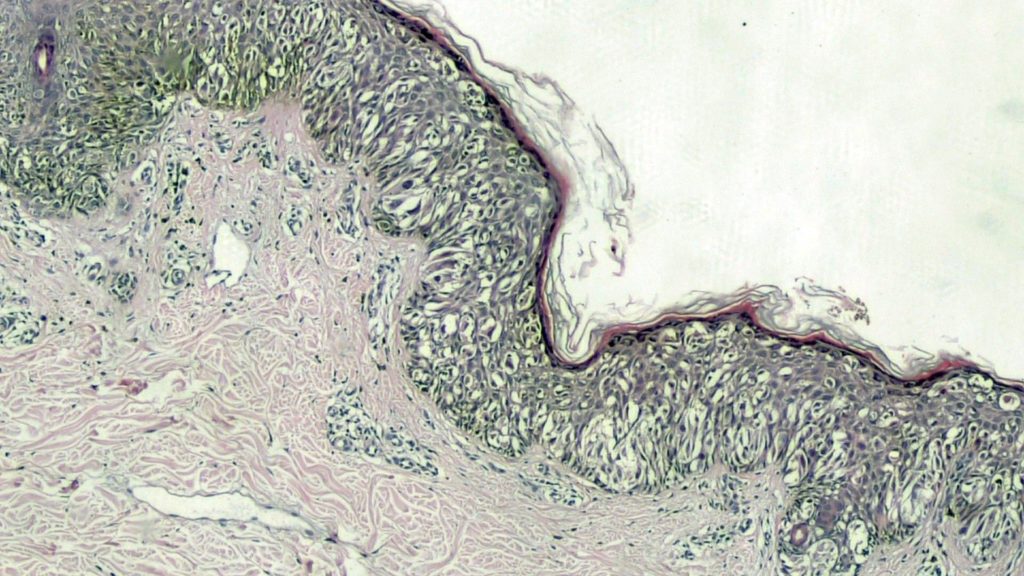
Acral Lentiginous Melanoma (ALM)
ALM is defined for the presence of a horizontal proliferative component characterized by a proliferation of atypical melanocytes, often with dendritic aspects, mainly localized to the basal layer of the epidermis and extended to the skin appendages associated with a lentiginous appearance with a marked elongation of the spurs epithelial. The vertical proliferative component is characterized by a proliferation of atypical melanocytes often type epithelioid, spindle or polymorphic; sometimes the cells can be arranged in bundles such as to simulate a Schwannian differentiation. More frequently it affects the plantar skin and the palmar skin, especially that of the thumb.
Nodular Melanoma (NM)
The term nodular melanoma defines a tumor characterized by the appearance of a nodule that develops rapidly without a pre-existing phase of horizontal growth.
Nodular melanoma appears to appear on an apparently normal skin. Although no lesion is observed existing or a horizontal phase, however there may be evidence of an association with a pre-existing nevus. Nodular melanoma accounts for about 10-12% of all types of melanoma in Caucasian subjects.
This neoplasm is by definition already growing vertically and is usually diagnosed at a fairly advanced stage and has a worse prognosis than other melanomas.
Lentigo Maligna Melanoma (LMM)
LMM occurs most frequently on the face and sun-exposed upper extremities of elderly people. Macroscopically, there is great variation in color, with tan-brown, black and even pink areas present. LMM can become an invasive malignancy (vertical growth phase) and it is characterized by thickening of the lesion with the development of elevated plaques or discrete nodules.
Histologically, LMM is characterized by an epidermal component of atypical melanocytes, singly and in nests, usually confined to the basal layer and with little pagetoid invasion of the epidermis.
Other histotypes of MM
Desmoplastic Melanoma (DM) is properly recognized as a variant of MM, with its distinct histological, immunophenotypic and molecular characterization.
The injury is usually considered 'scar-like' because, often, the first impression you have at low (panoramic) magnification of this injury is that of a scar.
At higher magnification it is possible to recognize fused cells, immersed in a desmoplastic stroma and focal with myxoid aspects, endowed with mild cytological characteristics, even if at times presenting a 'random' pleomorphism.
Rather characteristically DM cells are quite rich in mast cells and have a tendency to infiltrate the surrounding nerves. This variant of MM prefers the head/neck of older subjects, thus disproving it as a High Cumulative Solar Damage (H-CSD)-related melanoma.
Molecular studies have also confirmed a much higher rate of mutations for this lesion histotype than common MM variants.
Nevoid Melanoma (NM) is classically referred to as the 'tomb' of the dermatopathologist for its intrinsic ability, even in the eyes of a dermatopathologist with years of experience, to mimic a benign melanocytic nevus and, therefore, to pass as unknown. It is a rare type of MM, representing no more than 1% of all melanomas, which is commonly more frequent between the fourth and fifth decades of life, without a gender predominance.
Clinically, NM presents as a slowly growing lesion, most commonly on the proximal extremities, trunk, neck, and head.
From histological point of view, NM has an architectural pattern that looks very similar to that of a compound or intradermal nevus, with almost complete symmetry, good circumscription, and minimal or no pagetoid spreading.
The most important morphological characteristic distinguishing a NM from a standard nevus is the presence of a monomorphic population of small nevus-like melanic cells, present both in the superficial and deep portions of the dermis.
It is of absolute and indispensable importance to conduct very careful research of mitotic figures, able to make us suspect that we are in front of a NM.
Lascia un commento